- Best PCD Pharma Franchise Company
- +91 9216295095
- hcareindia@gmail.com
Adagrasib, an Antineoplastic Drug, New Molecule in Pharma
Data of pharmaceutical sector growth up to 2030
Best Remedy for Pigmentation
Table of Contents
ToggleAdagrasib, an Antineoplastic Drug, New Molecule in Pharma
Adagrasib (Brand Name: Krazati, by Mirati Therapeutics, Inc.) is an antineoplastic drug approved by the U.S. Food and Drug Administration (FDA) on December 12, 2022.
The FDA’s Center for Drug Evaluation and Research (CDER) approved a wide range of new drugs and biological products in 2022, many of which contain active moieties that had not been previously approved.
The list of new molecular entities and new therapeutic biological products approved by CDER in 2022 included approval for Adagrasib.
Adagrasib is a promising drug under development for the treatment of cancer. Also known as MRTX849, it is a small molecule that selectively inhibits the KRAS G12C mutation, which is found in several types of cancer. The drug works by interfering with the signaling pathway that drives the growth and survival of cancer cells.
Clinical trials have shown that Adagrasib has demonstrated significant anti-tumor activity in patients with advanced solid tumors harboring the KRAS G12C mutation. This has led to hope that Adagrasib could be a game-changer in cancer treatment and in the development of other targeted therapies in future.
Drug Class- Antineoplastic Agents
Trade Name- Krazati
Formula- C32H35C1FN7O2
IUPAC Name– 2-[(2S)-4-[7-(8-chloronaphthalen-1-yl)-2-[[(2S)-1-methylpyrrolidin-2-yl]methoxy]-6,8-dihydro-5H-pyrido[3,4-d]pyrimidin-4-yl]-1-(2-fluoroprop-2-enoyl)piperazin-2-yl]acetonitrile
Structure of Adagrasib
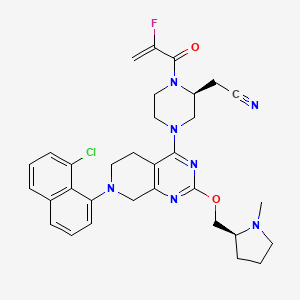
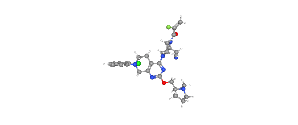
Adagrasib is an oral, highly selective, small-molecule, covalent inhibitor of KRAS G12C that is optimized to sustain target inhibition. This RAS GTPase family inhibitor is indicated for adult patients with KRAS G12C¬-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC) who have received at least one prior systemic therapy.
The FDA also approved the QIAGEN Thera screen KRAS RGQ PCR kit (tissue) and the Agilent Resolution ctDx FIRST Assay (plasma) as companion diagnostics for Krazati.
The approval was based on the KRYSTAL-1 trial, a single-arm, open-label clinical trial which included 116 patients with locally advanced or metastatic NSCLC with KRAS G12C mutations.
The main efficacy outcome measures were confirmed objective response rate (ORR) according to RECIST 1.1, as evaluated by blinded independent central review, and duration of response (DOR). The ORR was 43%, and median DOR was 8.5 months.
What are the common side effects of Adagrasib?
The most common side effects are:
- Diarrhea
- Nausea
- Fatigue
- Vomiting
- Musculoskeletal pain
- Hepatotoxicity
- Renal impairment
- Dyspnea
- Edema
- Decreased appetite
- Cough
- Pneumonia
- Dizziness
- Constipation
- Abdominal pain
- QTc interval prolongation
What is the recommended dose of Adagrasib?
The recommended adagrasib tablet dose is 600 mg orally twice daily until disease progression or unacceptable toxicity. The approval for Adagrasib is under accelerated approval based on overall response rate and duration of response.
Drug interactions may warrant dose modifications.
Studies Results
Studies of adagrasib have shown that the drug has a long half-life and extensive tissue distribution, and is well tolerated.
In clinical trials, adagrasib has also demonstrated CNS penetrance and single-agent responses in NSCLC, colorectal cancer, pancreatic cancer and other solid tumors with KRAS G12C mutations.
Preliminary results from the KRYSTAL-7 and KRYSTAL-1 trials suggest that the concurrent combination of adagrasib and pembrolizumab may provide a chemotherapy-free option for treatment-naive NSCLC with a manageable safety profile and encouraging clinical activity.
The FDA is currently evaluating the use of adagrasib to treat patients with non-small cell lung cancer harboring a KRAS G12C mutation and a new drug application has been accepted.
The most common side effects reported were diarrhea, nausea, fatigue, vomiting, musculoskeletal pain, hepatotoxicity, renal impairment and anemia.
*Source- NLM
**Also Read
